Heterogeneous Chemistry and Photochemistry of Mineral Oxides and Engineered Nanoparticles in Atmospheric Aerosol
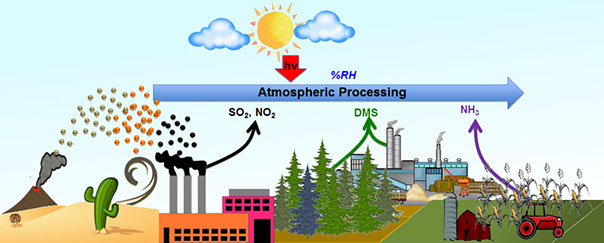
Atmospheric aerosol is largely composed of mineral dust, soil particles lifted into the atmosphere by wind action or volcanic eruptions, with an estimated annual emissions of 1,000-3,000 Tg/yr. This is expected to continue to increase as long as improper land-use practices. Conversely, with the emergence of new industries based on nanoscience and nanotechnology, it is expected elevated levels of engineered nanoparticles, manufactured particles in the size range of 1-100 nm, in atmospheric aerosol. Once air-bone, these aerosol particles provide reactive surfaces for chemistry and photochemistry in the presence of trace atmospheric gases (e.g. NO2 and SO2). Atmospheric processing of these mineral dust aerosol and manufactured nanoparticles plays an important role in the coupled global processes of chemistry, climate, biogeochemical cycles, and health.
In one of our such projects, we focus on chemical and photochemical transformation of aerosol dust particles in the presence of organic pollutants such as dimethyl sulfide (DMS) and ammonia (NH3) under atmospherically relevant conditions such as relative humidity, temperature and solar flux. These particular chemical and photochemical reactions are expected to acidify dust particles and alter reaction pathways and mechanisms.
Although field studies are matured and provide bulk information of these atmospheric reactions, there is still a great deal of fundamental research needed especially as the research relates to molecular speciation of adsorbates and surface coatings of particles,
quantifying rates of reaction and chemical processes as a function of relative humidity, temperature and solar flux, and determining the importance of these reactions relative to other pathways (e.g. homogeneous gas-phase reactions). In our lab, laboratory-based studies focus on mechanistic aspects of surface chemistry and photochemistry of atmospheric aerosol particles under simulated (controlled) environmental conditions. Thus, these studies are expected to provide molecular level insights to explain the field measurements and reaction kinetics to feed in to model studies.
ADMINISTRATIVE ASSISTANT
GAYAN R. RUBASINGHEGE
Associate Professor of Chemistry
New Mexico Institute of Mining and Technology
Department of Chemistry
801 Leroy Place
Socorro, NM 87801
Bethany Jessen
New Mexico Institute of Mining and Technology
Department of Chemistry
801 Leroy Place
Socorro, NM 87801
Phone: 575-835-5129
Fax: 575-835-5215
Phone: 575-835-5263
Fax: 575-835-5364
Copyright © 2018 The Environmental Chemistry Research Research Group. All rights reserved.